Explain the Difference Between Polar and Nonpolar Molecules
Which of the following molecules is more likely to dissolve in water. How is the polarity of a molecule related to its properties.

The Difference Between Polar And Nonpolar In Tabular Form
Classify the following diatomic molecules as polar or non-polar based on electronegativity difference.
. In all non-polar molecules it is not necessary that a nonpolar covalent bond is present. Electronegativity is the key factor that differentiates between polar and nonpolar bonds. A nonpolar molecule as a balanced or symmetrical distribution of charge whereas a polar molecule has an unequal charge distribution and has higher electron density on one side.
Up to 256 cash back CTQ-1. Polar molecules occur when there is an electronegativity difference between the bonded atoms. Explain the difference between a polar and a non-polar covalent bond.
Non-polar molecules do not have charges at their ends. In polar covalent molecules one or more than one polar covalent bond is present. Shape of these dielectrics are asymmetric.
A polar molecule always contains polar bonds but some molecules with polar bonds are nonpolar. Calculate the EN difference in the following molecules that involve Group VII. Pentane Hexane Carbon Dioxide.
An example of a polar molecule is. A polar molecule is a molecule that has oppositely charged regions as a result of polar bonds within the molecule. A molecule in which the bond dipoles present do not cancel each other out and thus results in a molecular dipolesee below.
Cancellation depends on the shape of the molecule or Stereochemistry and. Non-polar molecules are those whose center of gravity of nuclei coincide with that of the electronsFor polar molecules the centers of gravity are different for nuclei and the electronsThe dipole. How does polarity affect physical properties.
The electronegative difference between the atoms gives rise to polarity in the molecule. The geometry of atoms in polar molecules is such that one end of the molecule has a positive electrical charge and the other side has a negative charge. Shape of the dielectrics are symmetric.
Covalent bonds can be polar or non-polar. In chemistry it is the charge separation in a molecule that has atoms or groups of atoms with different. Non polar dielectrics are the non-polar compounds that do not conduct electricity.
Non polar dielectrics are non-polar. Explain the difference between a polar and a non-polar covalent bond. Polar vs non-polar molecules.
N2 PolarNon-polar O2 PolarNon-polar EN difference. Polar dielectrics are polar. The difference between polar molecules and nonpolar molecules.
Find step-by-step Chemistry solutions and your answer to the following textbook question. In polar molecules the difference in electronegativity among the bonding atoms results in the polarity of bonds on the other side. They are asymmetrical which means the polar bonds do.
A nonpolar molecule must have zero electric dipole moment which is possible if it contains nonpolar bonds OR polar bonds with dipoles that cancel. Explain why nonpolar molecules usually have much lower surface tension than polar ones. In non-polar molecules the difference of.
Mixing molecules of the same polarity usually results in the molecules forming a solution. For example CCl4 would be nonpolar because the dipoles all cancel and the electrical charge. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out.
Non-polar molecules are molecules that have no oppositely charge. The polarity of a compound refers to the property of having poles. Understanding electronegativity is essential to understanding polar and non-polar bonds.
Non polar molecules are symmetrical with no unshared electrons. Explain the difference between nonpolar molecules and polar molecules. Which of the following molecules is more likely to dissolve in water.
A polar molecule is formed between two dissimilar atoms but. 7 rows Within a molecule each polar bond has a bond dipole. Water HF CHCl 3.
Polar dielectrics are the polar compounds that do not conduct electricity. The main difference between polar molecules and nonpolar molecules lies in the arrangement of atoms in the molecule. The key difference between polar and nonpolar solvents is that polar solvents dissolve polar compounds whereas nonpolar solvents dissolve nonpolar compounds.
9 rows Difference between Polar and Nonpolar. A polar molecule must have polar bonds with dipoles that do not cancel. In simple terms polar means oppositely charged while non-polar means equally charged.
CzHOH or C2H6. H PolatNon-polar EN difference HCI PolarNon-polar EN difference. Polar and Non-Polar Molecules.

Image Result For Polar Vs Nonpolar Molecules Covalent Bonding Chemical Bond Chemistry Lessons
Difference Between Polar And Nonpolar Molecules Definition Formation Properties Examples
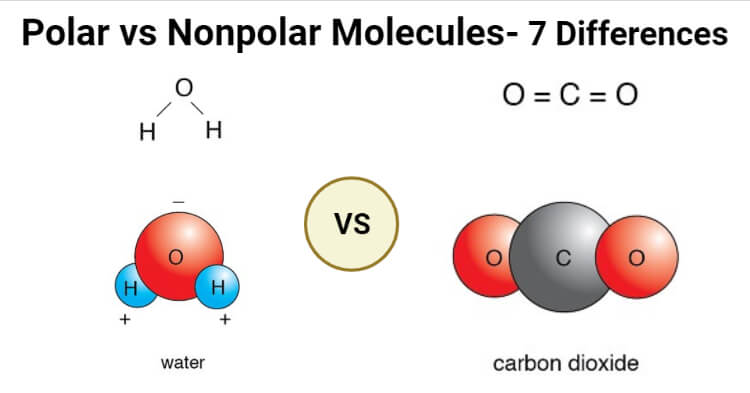
Polar Vs Nonpolar Molecules Definition 7 Key Differences Examples
No comments for "Explain the Difference Between Polar and Nonpolar Molecules"
Post a Comment